
応用生物プロセス学講座 > 研究内容 > ラッカーゼによる機能性食品素材の生産
ラッカーゼによる機能性食品素材の生産
Functional food production by laccase-mediated conversion of green tea catechins
We recently found that laccase (EC 1.10.3.2) was able to synthesize two benzotropolone derivatives, epitheaflagallin (5), and epitheaflagallin 3-O-gallate (6) (Fig. 1), from crude tea catechins in the presence of oxygen and gallic acid.1) These compounds have been previously synthesized from EGC (3)/EGCg (4) in the presence of catechol/pyrogallol by Takino and Imagawa.2) Using purified compounds including (1), (2), (3), (4), we confirmed that (5) and (6) were synthesized from (3) and (4), respectively, through laccase oxidation reaction with gallic acid. The reaction involves the preferential oxidation of gallic acid and the pyrogalloyl group (B ring) in (3) and (4) to the quinone intermediate galloquinone, followed by the Michael addition of the oxidized pyrogalloyl group in (3)/(4) and subsequent carbonyl addition across the ring and decarboxylation to yield benzotropolone skeletone. Recently, Sang et al. reported the enzymatic synthesis of tea theaflavin derivatives from catechin derivatives in the presence of hydrogen peroxide and peroxidase (EC 1.11.1.7).3) In peroxidase oxidation, the catechoyl/pyrogalloyl/galloyl groups in EC (1)/ECg (2)/EGC (3)/EGCg (4) are easily oxidized to form the quinone intermediates that subsequently yield the various theaflavin-related compounds. In our oxidation system, preferential conversion of (3) and (4) is possible, and very little EC (1) and ECG (2) reacted with the galloquinone intermediate in the reaction mixture. Laccase-dependent preferential oxidation of gallic acid and the pyrogalloyl group in (3) and (4) to the galloquinone intermediate makes it possible to convert (3) and (4) in crude tea catechins to (5) and (6).
To detect the presence of (5) and (6) in natural tea products, four kinds of black tea extract were analyzed. We confirmed that epitheaflagallin derivatives were minor components of all the black tea extracts tested. The black tea extracts contained a mean of approximately 0.1% (w/w) of (5) and (6) together.
Recently, many beneficial effects of green tea have been attributed to its abundant catechin, EGCg (4),4) and 3 O-gallate forms of catechins. We initiated research to elucidate the functions of epitheaflagallins in human health. During the course of our study, we found that (6) and TF 3-O-gallate (8) can inhibit pancreatic lipase. The laccase-treated green tea catechins, the purified (6) and (8) displayed the dose-dependent inhibition of pancreatic lipase.5) Although the effects of (6) (observed IC50: 1.2 mg/ml) and (8) (observed IC50: 0.4 mg/ml) were less dramatic than Xenical (an anti-obesity drug, observed IC50: 0.04 mg/ml), these compounds may still be suitable functional foods able to suppress the absorption of lipids during digestion. Interestingly, EGCg (4) and epitheaflagallin (5) had no effect on pancreatic lipase inhibition, indicating that the benzotropolone ring and the 3-O-gallate structure are necessary to inhibit pancreatic lipase. Epitheaflagallin 3-O-gallate (6) exhibited an inhibitory effect on lipid absorption in vivo in rats. As epitheaflagallins and TFs have a mild, black tea-like taste, a new tea drink rich in (6) and (8) could be popular for sale in Japan in the near future. Our research group has also confirmed the inhibitory effects of epitheaflagallins on α-glucosidases and on metastasis of human colon cancer.
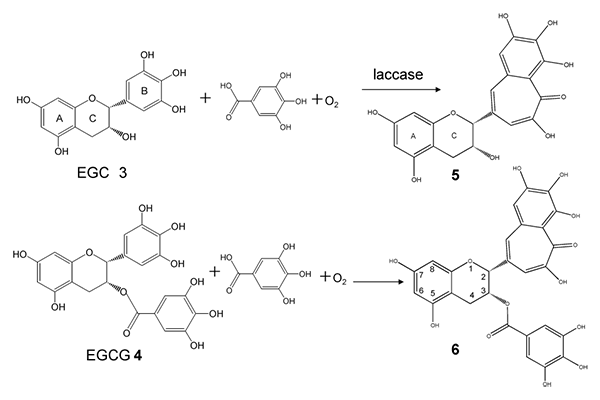
Fig.1 Reaction of EGC (3) and EGCG (4) with gallic acid by laccase-catalyzed oxidation.
References
- Itoh, N.; Katsube, Y.; Yamamoto, K.; Nakajima, N.; Yoshida, K. (2007) Laccase-catalysed conversion of green tea catechins in the presence of gallic acid to epitheaflagallin and epitheaflagallin 3-O-gallate. Tetrahedron, 63, 9488-9492.
- Takino, Y. & Imagawa. H. (1964) Studies on the mechanism of the oxidation of tea leaf catechins. Agric. Biol. Chem., 28, 125-130.
- Sang, S.; Lambert, J.D.; Tian, S,; Hong, J.; Hou, Z.; Ryu, J.-H.; Stark, R.E.; Rosen, R.T.; Huang, M.-T.; Yang, C.S; Ho, C.-T. (2004) Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med. Chem., 12, 459-467.
- Liao, A; Kao, Y.-H.; Hiipakka, R. A. (2001) Green tea: biochemical and biological basis for health benefits. Vitamins and Hormones, 62, 1-94.
- Itoh, N.; Katsube, Y. (2009) Biocatalytic conversion of green tea catechins to epitheaflagallinn, epitheaflagallin 3-O-gallate, and theaflavins: Production of promising functional foods. In Handbook of Green Tea and Health Research (Ed. by H. McKinly & M. Jamieson), Nova Science Publishers, pp.419.
我々の研究グループでは、市販のラッカーゼが、酸素と没食子酸の存在下で茶カテキンから2つのbenzotropolone骨格を有する化合物、即ちepitheaflagallin(5)とepitheaflagallin 3-O-gallate(6)(図1)を合成できることを初めて明らかにした1)。これらの化合物は、以前に滝野らがcatecholあるいはpyrogallolの存在下でEGC(3)及びEGCg(4)それぞれから化学合成したものであった。2) 化合物EC (1)、ECg (2)、(3)、(4)を反応基質として用いて検討したところ、没食子酸存在下でのラッカーゼによる酸化反応によって、(3)から(5)が、(4)から(6)がそれぞれ合成されることを確認した。図1に示したように、benzotropolone骨格が形成するメカニズムとしては、まず、没食子酸ならびに(3)または(4)のpyrogalloyl基がキノン中間体(galloquinone)に酸化され、次いで、キノン中間体同士のMichael付加が起こり、架橋構造を形成するカルボニル中間体を経て、脱炭酸が起こると推定している。
近年、Sangらは過酸化水素とペルオキシダーゼ(EC 1.11.1.7)の存在下で、茶カテキンからtheaflavin類の酵素合成を報告している。3)ペルオキシダーゼの反応では、EC(1)/ECg(2)/EGC(3)/EGCg(4)のcatechoyl/pyrogalloyl/galloyl基のすべてが容易に酸化されて、キノン中間体を生成し、種々のtheaflavin関連化合物が得られる。一方、我々の酸化反応系では、(3)と(4)が優先的に変換され、EC(1)やECg(2)は反応混合物中でgalloquinone中間体とほとんど反応しなかった。ラッカーゼ反応では、没食子酸ならびに(3)及び(4)のpyrogalloyl基がgalloquinone中間体へ優先的に酸化され、緑茶抽出物中の(3)及び(4)が、それぞれ(5)及び(6)に選択的に変換される。
機能性食品素材の開発においては、その素材の食経験が重要である。そこで、epitheaflagallin類についても、その存在を確認した。4種類の市販の紅茶抽出物(A.)で(5)と(6)を分析したところ、1つの試料を除いては、epitheaflagallin類が認められ、(5)と(6)は紅茶抽出物の微量成分であることが判明した。紅茶抽出物における(5)と(6)の含有率は、両者を合わせても約0.1%(w/w)程度であった。